News Categories
Disposable syringes

Disposable syringes
Safety Syringes/ Auto-Disable Syringes
Safety Syringes/ Auto-Disable Syringes
Oral/Enteral Syringes
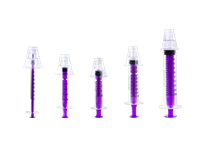
Oral/Enteral Syringes
Infusion Sets

Infusion Sets
Urine Bags

Urine Bags
Urine Cups

Urine Cups
the medical products inspect process for disposable syringe
1. Biocompatibility test report
The biocompatibility of the device was evaluated according to EN ISO 10993-1, where the tests items are chosen accordingly. The evaluation and test results shows that benefits weighing against risks, the biocompatibility of the device is considered to be suitable for intended use. Detailed biocompatibility evaluation is provided in attachment # LL/QS9.2/01/1301.
Based on the test results of type examination report for sterile hypodermic syringes for single use issued by competent authority, CFDA Jinan Medical Equipment Inspection Institute, concerning cytotoxity, irritation, sensitization, systemic toxicity(acute) and Haemocompatibility it is concluded that the biocompatibility of the product is in line with essential requirement from MDD.
2. Physical and chemical properties
The physical and chemical properties have been defined in product specification which are raised from design output and Essential Requirements.
The type test reports of finished products concerning physical and chemical properties are provided in attachmen。
3. Usability evaluation
The hazards and hazardous situation related to the usability have been taken into consideration during risk management process, and the mitigation measures are documented in risk management file. Usability engineering of the device is conducted according to EN 62366 and the result is documented in Usability Evaluation Report. Refer to attachment # LL/QS9.2/01/1302.
4 .Packaging evaluation
The products are packaged in sterile barrier packaging system which aims to ensure the sterile condition of the products inside the packaging within specified shelf life. The primary package is paper-polypouchandplastic/blisterbox sealed by heat-sealing machine.
The primary packaging process is evaluated and validated according to EN ISO 11607-1/2. A validation group is established to coordinate and implement the validation, following the validation procedure.
Validation protocol has been established and implemented by the validation group. Validation report has been reviewed, approved and signed by the validation group. According to the validation result, routine operation and control work instruction for primary packaging has been established in work instruction.
The processes will be revalidated if changes are made to the equipment, product, packaging materials or packaging processes and etc., which compromise the original validation and affect the sterility, safety or efficacy of the sterile products. Annual revalidation or reviews will also be performed and documented to evaluate the efficacy of the previous validation result.
Refer to attachment
Packaging qualification validation report of Sterile hypodermic syringes for single use #LL/WI7.5.7b/03
Shelflife validation report of sterile hypodermic syringes for single use #LL/WI7.5.7b/05/01
5. Sterilization
The product is supplied sterile, and sterilized by EO sterilization to ensure SAL≥ 10 -6. The EO sterilization is conducted in-house.
We has established a QM system according to EN ISO 13485 for provision of EO sterilization service of medical device according to EN ISO 11135, and under annual surveillance audit by the Certification Body.
The sterilization process has been validated according to EN ISO 11135, which has thereby determined the routine control and monitoring parameters. The validation and re-validation is conducted according to EO sterilization validation procedure, and the result is documented in EO sterilization validation report.
For routine release of sterilization, the sterilization certificate will be provided for each sterilization batch, reviewed and approved by Quality Department.
Sterilization validation report please refer to attachment #LL/WI7.5.7a/02/02



Quick Response within 24 hours!
Tel: (+)86-519-88168398
WhatsApp: +86-13961432323
Email: sale01@lelun.com
HengShanQiao Town, out of East Gate, Changzhou ,Jiangsu, China
Changzhou Medical Appliances General Factory Co., Ltd. was built in 1988, it is a modern factory specialized in producing the disposable medical appliances in China. The factory is only 1km to Hengshan entrance of Huning high-speed road and is about 20 miles to Changzhou airport. So the traffic is convenience.
The area of the factory is 40000㎡, the area of purifying workshop is 7000m, and fixed assets are about 5,000,000USD. Our main products are Disposable infusion sets, Disposable blood transfusion sets, Disposable Sterile syringe sets, latex glove, disposable infusion set, simple oxygen mask, nebulizer mask, urine cup, hernia mesh, urine bag etc. Now we can manufacture more than 200,000,000 sets per year.